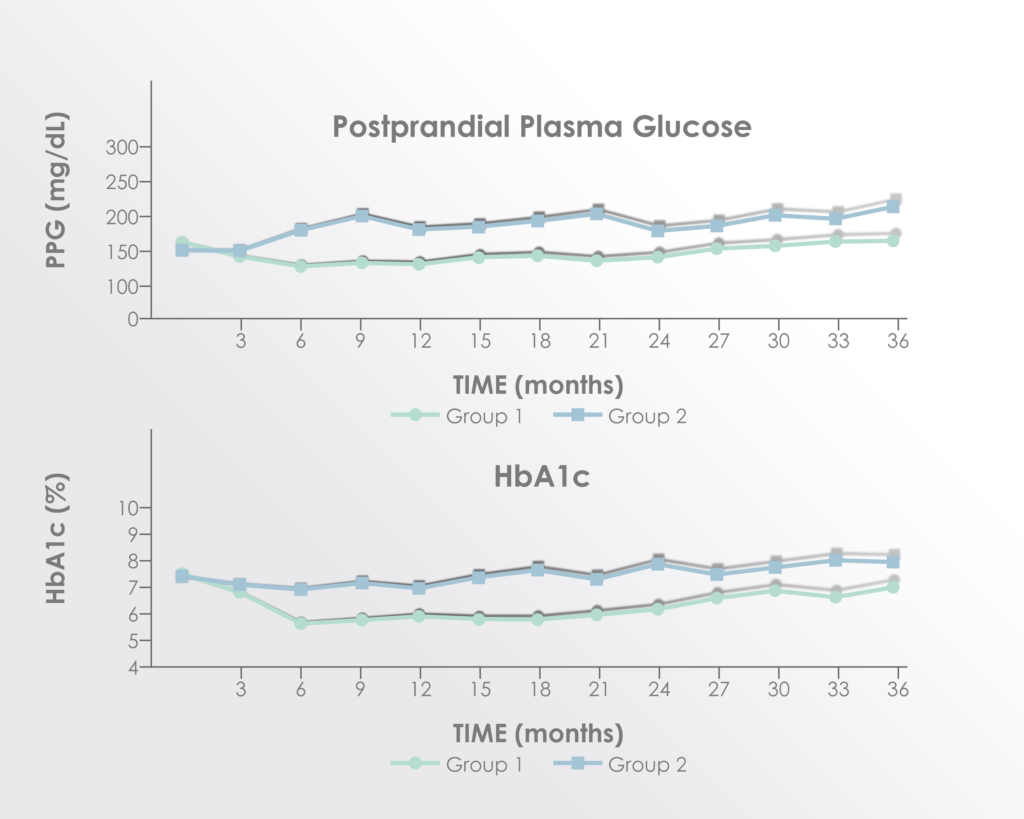
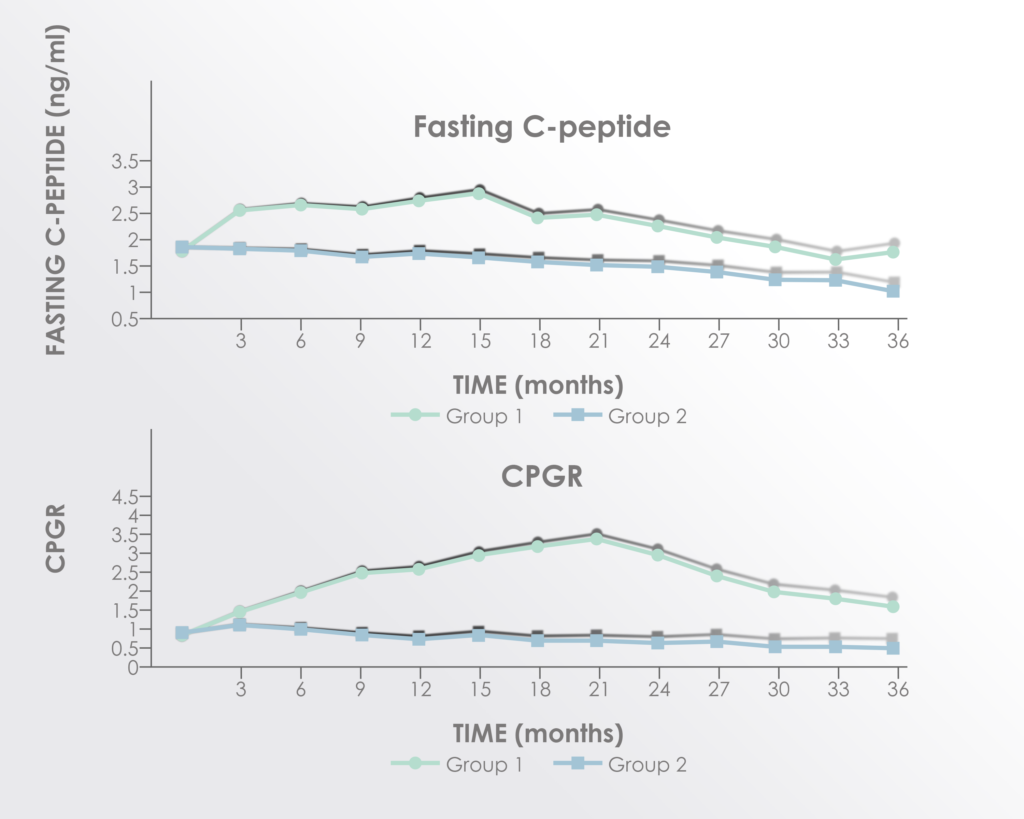
Neuropathic pain, also known as nerve pain or neuralgia, occurs when there is damage to the nerves; it is one of the major consequences of spinal cord injury. In this study based in Spain, 10 patients received a dose of 100 million bone marrow MSCs, repeated at 4 months and 7 months. No adverse events were reported during the 10-month follow-up period. 9 out of 10 of patients reported relief in neuropathic pain. Their mean pain scores significantly decreased from 5.50 to 1.50, as measured by standard pain scales. This shows that MSC therapy (particularly in repeated doses) is a safe and potentially beneficial treatment option for spinal cord injury patients suffering from neuralgia.
Intrathecal administration of autologous bone marrow stromal cells improves neuropathic pain in patients with spinal cord injury.
Neuropathic pain (NP) is highly disabling, responds poorly to pharmacological treatment, and represents a significant cause of decreased quality of life in patients suffering from spinal cord injury (SCI). In recent years, cell therapy with autologous mesenchymal stromal cells (MSCs) has been considered as a potential therapeutic weapon in this entity. Ten patients suffering chronic SCI received 100 million MSCs into subarachnoid space by lumbar puncture (month 1 of the study) and this procedure was repeated at months 4 and 7 until reaching a total doses of 300 million MSCs. Intensity of NP was measured by standard numerical rating scale (VAS) from 0 to 10, recording scores previous to the first MSCs administration and monthly, until month 10 of follow-up. Months 1, 4, 7 and 10 of the study were selected as time points in order to a statistical analysis by the nonparametric Wilcoxon rank test. Our results showed significant and progressive improvement in NP intensity after the first administration of MSCs (p: 0.003). This study supports the benefit of intrathecal administration of autologous MSCs for the treatment of NP in patients with SCI.