While updating my database of human clinical trials using expanded human umbilical cord mesenchymal cells I came across a new article (April, 2016) describing a 36-month safety in subjects given intravenous cells for in a type 2 diabetes study. The study, a phase I/II, 36‑month, randomized controlled trial was conducted in patients diagnosed with T2DM according to the criteria outlined by the American Diabetes Association and performed at Qingdao University by Dr. Jianxia Hu et. al. 1 Not only were there no adverse events in the treatment group of 31 subjects but there were significant improvements in blood sugar control and decreased diabetes-related complications.
Two intravenous infusions of expanded human umbilical cord mesenchymal stem cells were given four weeks apart. The dose of each infusion was based on the weight of the subjects and averaged 60 million cells.
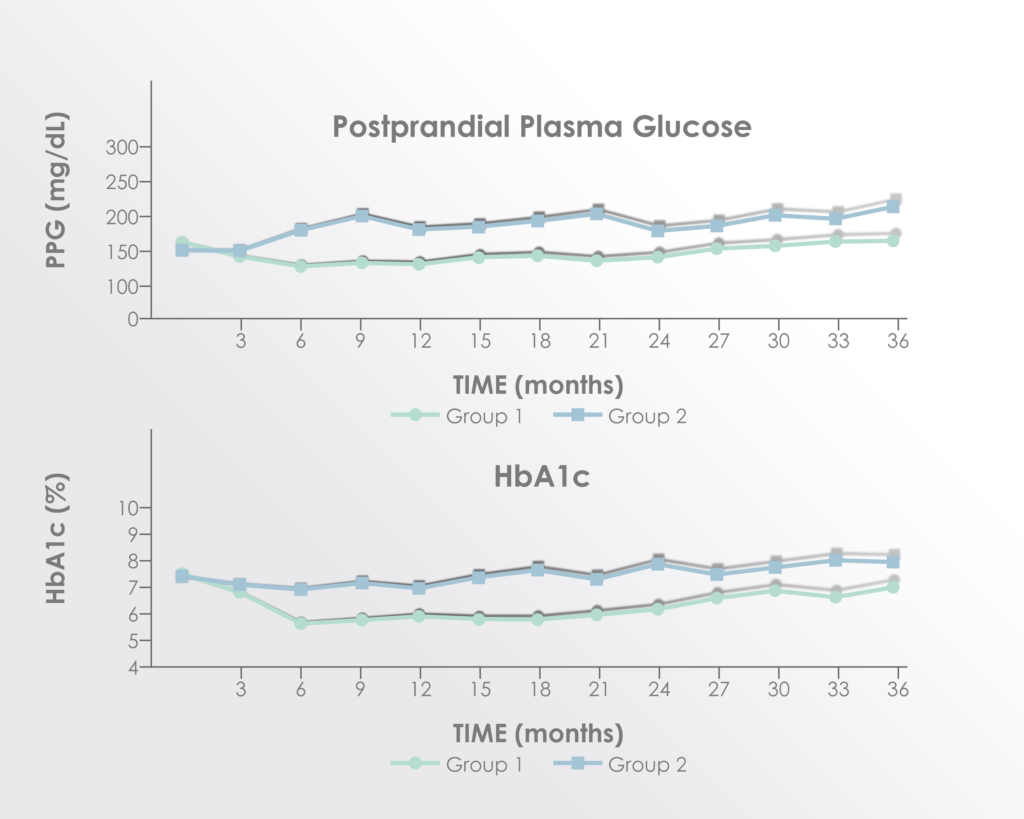
Significant improvements were seen in post-prandial glucose and hemoglobin A-1C levels but not fasting glucose.
Adapted from Figure 2 of Hu et al. 2016 Long term effect and safety of Wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes.Exp Ther Med. 2016 Sep; 12(3): 1857–1866 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4997981/
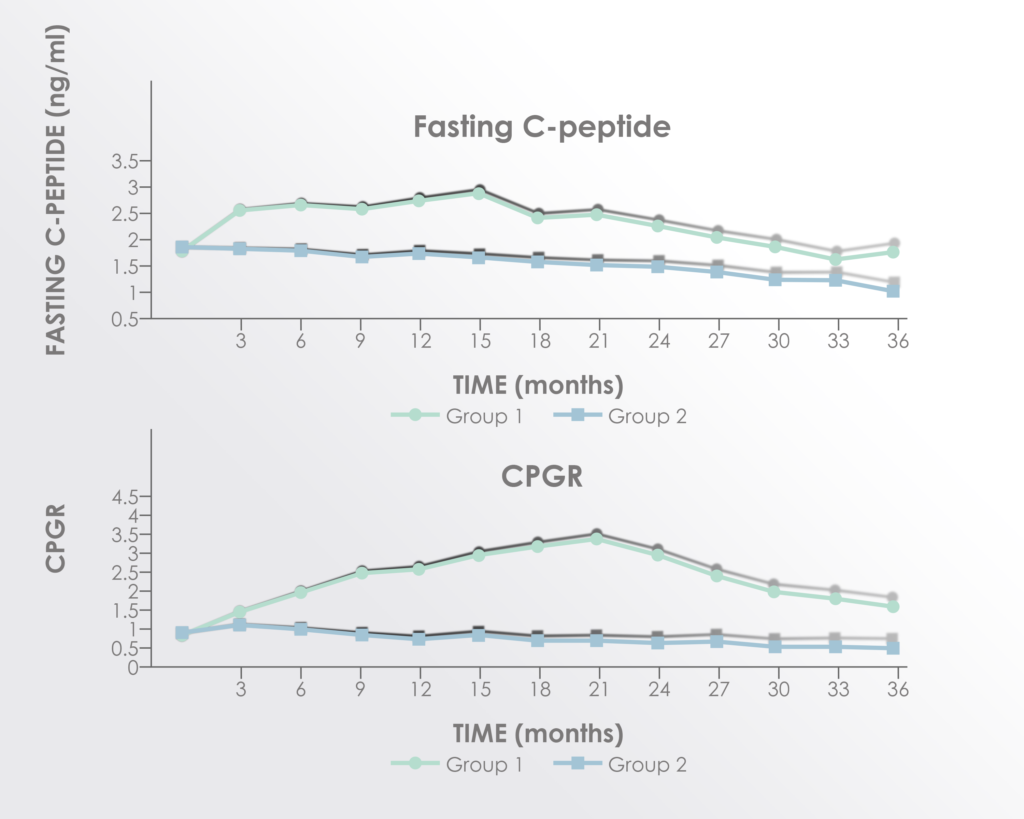
C-peptide (surrogate for endogenous insulin production) and C-peptide to glucose ratio were both significantly improved in the treatment group.
Adapted from Figure 3 of Hu et al. 2016 Long term effect and safety of Wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes.Exp Ther Med. 2016 Sep; 12(3): 1857–1866 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4997981/
Significant improvement was seen in the HOMA‑β, homeostasis model assessment of pancreatic islet β‑cell function; while there was no improvement in the HOMA‑IR, homeostasis model assessment of insulin resistance.
The improvements seemed to peak between 15 and 21 months after treatment supporting a rationale for re-treatment before 15 months.
- JIANXIA HU, YANGANG WANG, HUIMIN GONG, CHUNDONG YU, CAIHONG GUO, FANG WANG, SHENGLI YAN and HONGMEI XU. Long term effect and safety of Wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes. EXPERIMENTAL AND THERAPEUTIC MEDICINE 12: 1857-1866, 2016. Stem Cell Research Center, The Affiliated Hospital of Qingdao University, Qingdao, Shandong 266003